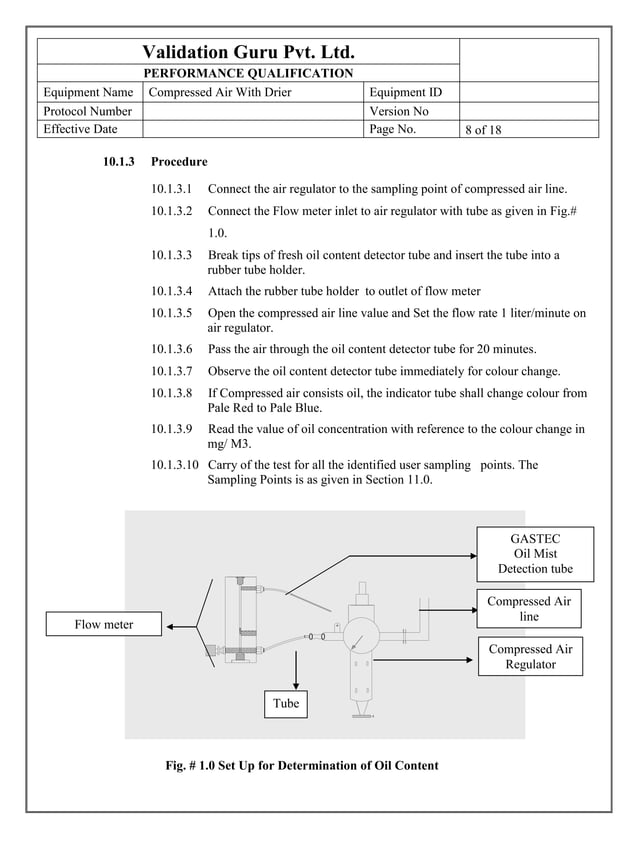
What Is Compressed Air Validation . contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water.
from www.slideshare.net
this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water.
Protocol for compressed air validation PDF
What Is Compressed Air Validation The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. this part of iso 8573 provides general information about contaminants in compressed air systems as well as links.
From www.scribd.com
Complete Solution For Your Compressed Air Validation PDF What Is Compressed Air Validation the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. this part of iso 8573 provides general information about contaminants in compressed air systems as well as. What Is Compressed Air Validation.
From www.scribd.com
Presentation On Validation of Compressed Air PDF Verification And What Is Compressed Air Validation this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. contamination in. What Is Compressed Air Validation.
From www.scribd.com
Compressed Air Validation PDF Gases Energy Technology What Is Compressed Air Validation — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. this part of. What Is Compressed Air Validation.
From www.youtube.com
Compressed air Qualification Compressed air Validation What Is Compressed Air Validation compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. — when. What Is Compressed Air Validation.
From www.safewellsolutions.co.uk
Compressed Air Quality Testing & Validation in accordance with ISO 8573 What Is Compressed Air Validation this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. — compressed air validation. What Is Compressed Air Validation.
From slideplayer.com
A Seminar on VALIDATION OF COMPRESSED AIR ppt video online download What Is Compressed Air Validation this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. the validation of. What Is Compressed Air Validation.
From reliable.world
Compressed Air Validation NABL Certified Calibration Services Pune What Is Compressed Air Validation this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. contamination in a. What Is Compressed Air Validation.
From airtesting.com
Compressed Air Testing Services TRI Air Testing Inc. What Is Compressed Air Validation — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. this part of iso 8573 provides general information about contaminants in compressed air systems as well as links.. What Is Compressed Air Validation.
From dokumen.tips
(PPT) Validation of Compressed Air DOKUMEN.TIPS What Is Compressed Air Validation the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. contamination in. What Is Compressed Air Validation.
From www.indiamart.com
Compressed Air Validation at best price in Vadodara ID 22495506662 What Is Compressed Air Validation this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. The compressed air. What Is Compressed Air Validation.
From caloxinc.com
All About Compressed Air Uses, Storage, and Best Practices What Is Compressed Air Validation the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. compressed air. What Is Compressed Air Validation.
From www.indiamart.com
Compressed Air Quality Validation Services at best price in Hyderabad What Is Compressed Air Validation the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. compressed air. What Is Compressed Air Validation.
From www.slideshare.net
Protocol for compressed air validation What Is Compressed Air Validation — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. — when performing a validation of a new or modified compressed air system, identify the specification requirements,. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. contamination in a. What Is Compressed Air Validation.
From jhfoster.com
Ensure Compressed Air Purity Levels Using ISO JHFOSTER What Is Compressed Air Validation contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. . What Is Compressed Air Validation.
From www.slideshare.net
Protocol for compressed air validation What Is Compressed Air Validation this part of iso 8573 provides general information about contaminants in compressed air systems as well as links. contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements. What Is Compressed Air Validation.
From www.scribd.com
Compressed Air Filter Performance Validation Brochure PDF What Is Compressed Air Validation contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. compressed air is widely used throughout industry, with over 90% of manufacturing industries globally using compressed air. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and. . What Is Compressed Air Validation.
From usvalidation.com
Validation of Compressed Air Systems What Is Compressed Air Validation contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. — compressed air validation is recorded evidence that such parameters as aerosol particle content, dew point, liquid water. this part of iso 8573 provides general information about contaminants in compressed air systems as well as links.. What Is Compressed Air Validation.
From www.slideshare.net
Validation of compressed air and nitrogen PPT What Is Compressed Air Validation contamination in a compressed air system originates from many sources and is not related solely to the compressor or it’s lubricants. the validation of a compressed air system in the pharmaceutical industry is typically performed according to the requirements and. — when performing a validation of a new or modified compressed air system, identify the specification requirements,.. What Is Compressed Air Validation.